Installation, Commissioning and Qualifications
The Best for the Best
With the right teams, we derive the best performances. Our go-to strategy for delivering the best results is Rebuild, Execute and Recast.
To make a client’s project successful, rich industrial expertise is crucial. Clean and Control’s commissioning, qualification and validation teams have decades of experience in handling pharmaceutical projects and equipment. We ensure all the projects are executed smoothly and by GMP guidelines. With our industry-centric knowledge, we identify bottlenecks and resolve them even before they cause any loss to the client. For successful project execution, our experts ensure compliance with international and local regulations.
We implement a reliable audit and quality management system to resolve issues before they become gigantic. Our timely and speedy corrections lead us to the right methodology for executing, commissioning, and validating the pharmaceutical facility, completing a crucial link in the turnkey pharmaceutical engineering process.
We are equipped with various international guidelines for the installation and commissioning of pharmaceutical facilities including ASTM E-2500, ISPE, and CGMP.
Clean and Control stages of various services include
- A strategic plan with a defined sequence of installation activities.
- Preparation of the detailed specification records
- Team of industrial experts.
- We cover structural progress every day, feedback tools & perform a detailed revision of course procedures.
- Field statements & reports.
- Phase-wise commissioning.
- Installation of data recording & commissioning tools.
- Monitoring and testing
- Validation


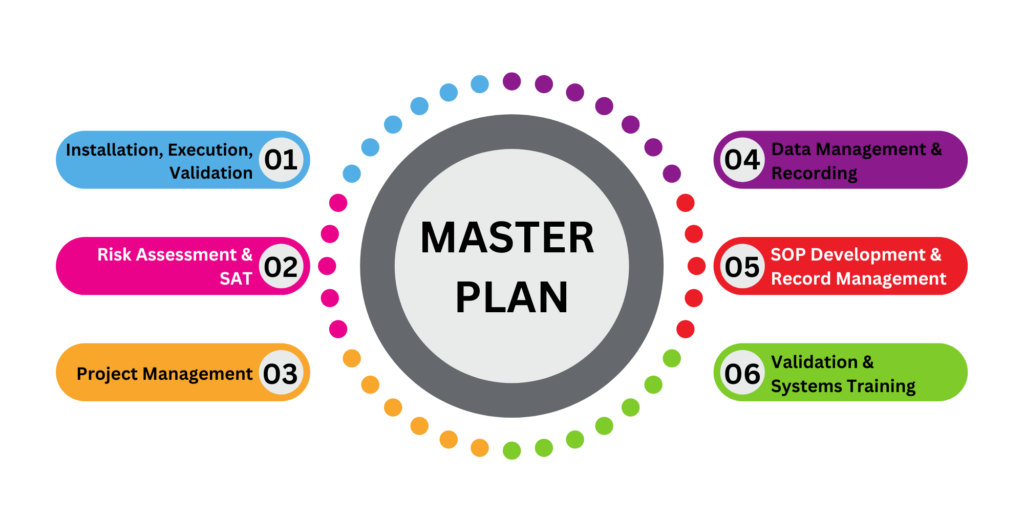
As a part of this activity, we provide a validation master plan and support to our clients until they have fully validated their facilities.
In addition to verifying proper installation, start-up, functional performance and turnover, our team also provides commissioning documents. To ensure all the systems are in a specified condition, our commissioning services include SAT (Site Acceptance Test).
Clean and Control Extended Services:
- Risk assessment
- SAT preparation and conclusion
- Documentation
- Project management
- SOP development
- Calibration and testing
- Training
From Vision to Execution